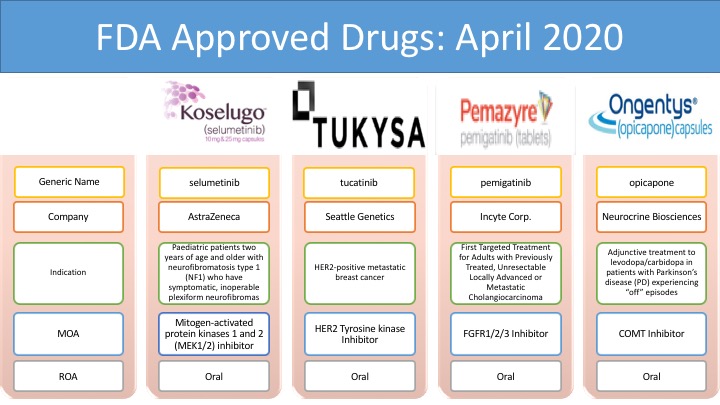
Koselugo (selumetinib): AstraZeneca plc and Merck & Co., Inc.
Koselugo (selumetinib) is the first drug approved by the FDA for the treatment of pediatric patients, 2 years of age and older, with neurofibromatosis type 1 (NF1), a genetic disorder of the nervous system causing tumors to grow on nerves. The drug is an inhibitor of mitogen-activated protein kinases 1 and 2 (MEK1/2). Koselugo was granted with many designations: US FDA Breakthrough Therapy Designation in April 2019, Rare Pediatric Disease Designation in December 2019, Orphan Drug Designation in February 2018, EU orphan designation in August 2018 and Swissmedic Orphan Drug Status in December 2018 for the treatment of pediatric patients with NF1 PN.
Tukysa (tucatinib): Seattle Genetics
Tukysa (tucatinib) is an oral, small-molecule tyrosine kinase inhibitor (TKI) of HER2, a protein that contributes to cancer cell growth. The drug is approved in combination with trastuzumab and capecitabine for the treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting. Tukysa was approved by different regulatory bodies such as the US Food and Drug Administration (FDA) in collaboration with Health Canada, Australian Therapeutic Goods Administration (TGA), Swissmedic (SMC, Switzerland), and Health Sciences Authority (HSA, Singapore) in April 2020.
Pemazyre (pemigatinib): Innovent Biologics, Inc.
Pemazyre (pemigatinib) is the first and only FDA-approved treatment for the treatment of adults with previously treated, unresectable locally advanced, or metastatic cholangiocarcinoma. Pemazyre is a potent, selective, oral inhibitor of Fibroblast growth factor receptor (FGFR) 1, 2, and 3. The drug is marketed by Incyte in the United States. Incyte has granted Innovent Biologics rights to develop and commercialize pemigatinib in hematology and oncology in Mainland China, Hong Kong, Macau, and Taiwan. Incyte has retained all other rights to develop and commercialize pemigatinib outside of the United States.
Ongentys (opicapone): Neurocrine Biosciences, Inc.
Ongentys (opicapone) is the first and only approved Catechol-O-methyltransferase (COMT) inhibitor indicated for the treatment of Parkinson’s disease with off episodes, used as an adjunctive treatment to levodopa and carbidopa. It was developed by Portuguese pharmaceutical group BIAL. Neurocrine Biosciences entered an exclusive licensing agreement with BIAL for the development and commercialization of opicapone in North America in September 2017. BIAL is currently responsible for the marketing of Ongentys in the United Kingdom, Italy, Spain, Germany, and Portugal.
I have been looking for this information for quite some times. About four and a half hours of continuous searching, at last I found that in your web site. I wonder why Bing never display this type of useful websites in the top of the list. Usually the first few websites are junks. Maybe its time to change to another search engine.
I was reading throught some of the posts and i locate them to be altogether attention-grabbing. sorry my english is not exaclty the actually best. would there be anyway to transalte this into my vernacular, spanish. it would in reality better me a lot. since i could compare the english lingo to the spanish language.
I would like to voice my gratitude for your kindness in support of people that should have assistance with this important area. Your personal dedication to getting the solution along became exceedingly productive and have truly empowered women like me to get to their pursuits. Your important useful information indicates this much a person like me and far more to my office colleagues. Regards; from everyone of us.
I have been looking for this information for quite some times. About four and a half hours of continuous searching, at last I found that in your web site. I wonder why Bing never display this type of useful websites in the top of the list. Usually the first few websites are junks. Maybe its time to change to another search engine.
I am incessantly thought about this, thankyou for putting up.
I wasnt aware of the many ripples and depth to this story until I surfed here through Live search! Good job.
Dude, I must say that overall I am fairly taken with this web page. It is apparent that you know you subject matter and you are passionate about it.
First Off, let me commend your clearness on this subject. I am not an expert on this subject, but after reading your piece of writing, my understanding has developed substantially. Please allow me to grab your rss feed to stay in touch with any forthcoming updates.
I like that site layout ! How was it made? Its really good.
Hey 🙂 Just between, are some totally uncorelated websites blogs to ours, however, they are ultimate worth checking order out..
Trusting to make the right decisions can be tough. It can take many people a long time to build a strong moral system. Its not the sort of thing that simply just happens.
Took me time to read all the comments, but I really enjoyed the article. It proved to be Pretty helpful to me and I am positive to all the commenters here It is always nice when you can not only be informed, but also entertained Im positive you had fun writing this post.
I have wanted to post something like this on my website and you have given me an idea. Cheers.
Have you ever thought about adding a little bit more than just your thoughts? I mean, what you say is important and everything. But its got no punch, no pop! Maybe if you added a pic or two, a video? You could have such a more powerful blog if you let people SEE what youre talking about instead of just reading it.
Excellent read, I just passed this onto a friend who was doing a little research on that. And he actually bought me lunch because I located it for him smile So let me rephrase that: Thanks for lunch!
You have actually created some exceptional points here. I specifically appreciate the way you’ve been able to stick so much thought into a relatively short post (comparitively) which creates it an thoughtful publish on your subject. In my opinion, you’ve presented the topic in a quite thorough yet concise manner, that is genuinely useful when somebody wants to get the facts without spending too a lot time searching the web and sifting out the noise to discover the answers to their questions. I usually get so frustrated with so plentiful in the final results inside the major SE’s due to the fact they normally seem to mostly be filled with filler content that often isn’t extremely sensible. If you don’t mind I’m going to add this post and your webpage to my delicious favorites so I can share it with my family. I appear forward to approaching back to read your future posts as well.