Novel Coronavirus (COVID-19) Preventive Measures
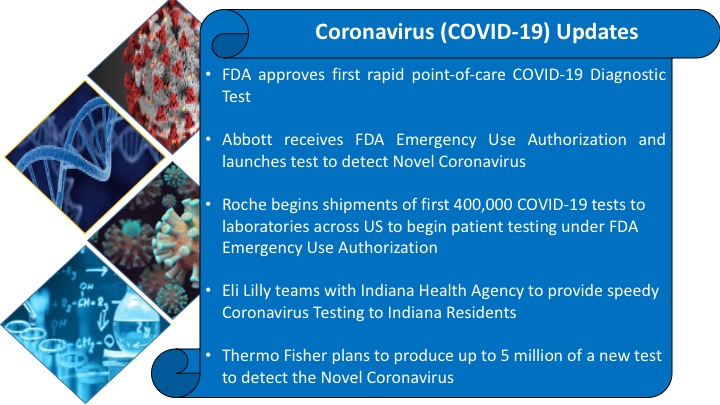
The novel Coronavirus (COVID2019) outbreak identified in China is causing global concern. It has spread to more than 200 countries worldwide. According to the World Health Organization (WHO)’s live report, there are more than 13,00,000 confirmed cases of the novel Coronavirus (COVID-19) outbreak. Approximately, 74,306 number of people died due to the novel Coronavirus. The United States accounts for highest cases (363,321) followed by Spain (135,032), Italy (132,547), Germany (99,225) China (83,157) and France (73,488).
There are no specific vaccines or treatments for COVID-19. Several clinical trials are undergoing for evaluating the potential treatments. Hence, it is essential to prevent Coronavirus infection and slowing the rate of transmission.
“Prevention is better than cure”
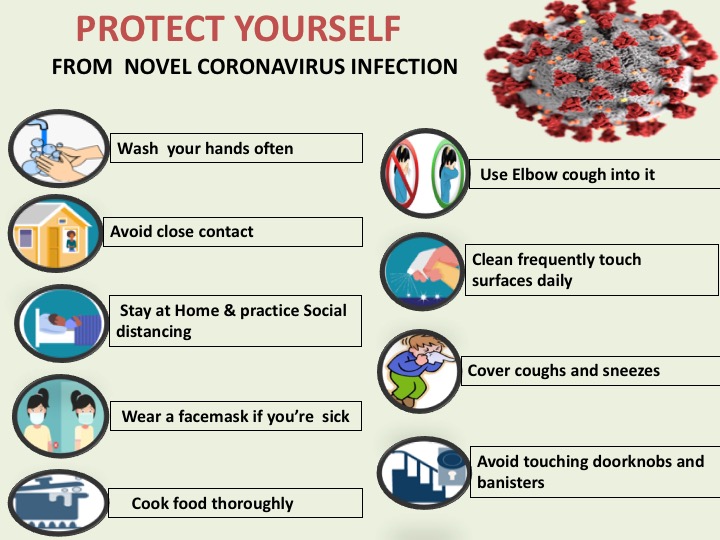
Preventive measures for Coronavirus are as follows:
- Wash Your Hands
Wash your hands regularly with soap and water, or clean them with alcohol-based hand rub
- Avoid Close Contact
Maintain at least 1 metre distance with the people coughing or sneezing
- Social Distancing & Stay Home
Stay home and Practice physical distancing by avoiding unnecessary travel and staying away from large groups of people
- Wear Facemask
Wear a facemask if you are sick
- Cook Food
Cook food thoroughly
- Elbow Coughing
Use Elbow to cough into it
- Cleanliness
Clean frequently touch surfaces daily
- Cover coughs and sneezes
Cover your mouth and nose when coughing or sneezing
- Avoid touching surfaces
Avoid touching doorknobs and banisters