Multiple sclerosis (MS) is a condition that can affect the brain and spinal cord, causing a wide range of potential symptoms, including problems with vision, arm or leg movement, sensation, or balance. It is a chronic autoimmune inflammatory disease of the central nervous system that damage and scars the sheath, and potentially the underlying nerves. Multiple sclerosis is affecting 2.3 million people worldwide, with females more affected than males.
According to the National Institute for Neurological Disorders and Stroke (NINDS), approximately, 250,000-350,000 people are suffering from the Multiple Sclerosis in the United States. The majority of the people with multiple sclerosis are diagnosed between the ages of 20 and 50, with at least two to three times more women than men being diagnosed with the disease. The most common symptoms associated with MS include fatigue, difficulty walking, vision problems, problems controlling the bladder, etc. The average life expectancy is slightly reduced for people with MS. The progress, severity, and specific symptoms of MS in any one person cannot yet be predicted.
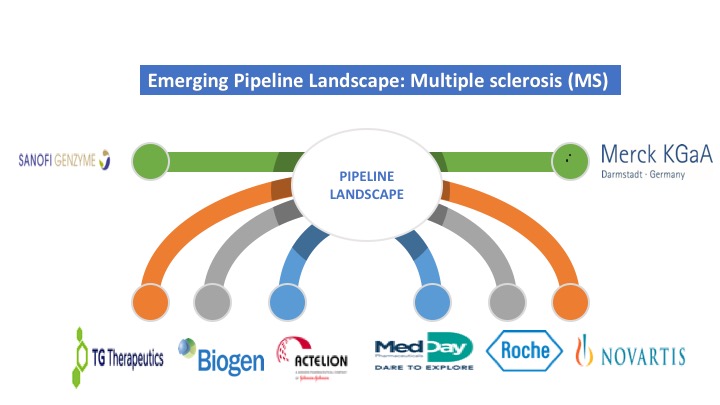
There are various diversify companies involved in the discovery and development of a potentially robust clinical pipeline for the treatment of Multiple Sclerosis. Merck Healthcare KGaA, Novartis, Sanofi, TG Therapeutic, Biogen, MedDay Pharmaceuticals SA, and Hoffmann-La Roche are the major market players
Actelion (Ponesimod)
Ponesimod is an investigational selective sphingosine-1-phosphate receptor 1 (S1P1) modulator that inhibits S1P protein activity and reduce the number of circulating lymphocytes that can cross the blood-brain barrier. The Janssen Pharmaceutical Companies of Johnson & Johnson (Janssen) had submitted the New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for ponesimod for the treatment of adult patients with relapsing multiple sclerosis (MS).
Merck Healthcare KGaA (Evobrutinib)
Evobrutinib is an investigational oral inhibitor of Bruton’s tyrosine kinase (BTK) which is thought to be important in the development and functioning of various immune cells including B lymphocytes and macrophages. Currently, clinical research study is ongoing on Evobrutinib. The study would evaluate the efficacy and safety of evobrutinib administered orally twice daily versus Teriflunomide (Aubagio), administered orally once daily in participants with Relapsing Multiple Sclerosis (RMS).
Hoffmann-La Roche (Ocrelizumab)
Ocrelizumab is a humanized monoclonal antibody that selectively targets the CD20-positive B-cells implicated in the inflammatory and neurodegenerative processes of multiple sclerosis (MS), to effectively impact disease progression while maintaining immunosurveillance.
A clinical trial of Phase-III study is being conducted to treat MS. This is a single arm, open label, multicenter extension study in participants on ocrelizumab therapy at the end of Treatment period of the Roche P-trial. Participants would receive the treatment with ocrelizumab as single 600 mg infusions every 24 weeks for two years.
TG Therapeutics, Inc. (Ublituximab)
Ublituximab (TG-1101) is a monoclonal antibody that targets CD20 positive B-cells, a component of the body’s immune system. When it attaches to the B-cell it triggers immune reactions (including antibody-dependent cellular cytotoxicity, ADCC, and antibody-dependent cellular phagocytosis that result in the death of the targeted B-cell).
While ublituximab is a novel patented molecule, it’s mechanism of action is similar to the approved anti-CD20 monoclonal antibodies. Ublituximab has been bioengineered to remove certain sugar molecules from the anti-CD20 antibody, which are naturally occurring. The removal of the sugar molecules in a process called glycoengineering have enhanced the potency of ublituximab with data showing 50-100x greater activity than non-bioengineered anti-CD20 antibodies.
MedDay Pharmaceuticals SA (MD1003)
MD1003 is a highly concentrated oral formulation of biotin currently under clinical investigation as a treatment for primary and secondary progressive multiple sclerosis (PPMS and SPMS). It is being developed by MedDay. It is a promising treatment that exhibits the pro-myelinogenic effects and enhances the supply of energy for nerve impulse transmission.
Biogen (Opicinumab)
Opicinumab is a human monoclonal antibody that targets LINGO-1, a protein that suppresses the redevelopment of axons (brain cells that send functional information throughout the body) and re-formation of myelin sheaths (fibers that protect the axons). Axons and myelin sheaths are lost or damaged in patients with MS, leading to loss of physical and cognitive function. By blocking LINGO-1, opicinumab is formulated to promote regeneration of axons and myelin.
The Phase II, double-blind AFFINITY study is assessing the effectiveness of opicinumab as an add-on therapy in people with relapsing or secondary-progressive forms of MS (RMS or SPMS).
Novartis (Ofatumumab)
Ofatumumab is a fully human anti-CD20 monoclonal antibody (mAb) self-administered by a once-monthly subcutaneous injection in development for RMS. Ofatumumab works by binding to the CD20 molecule on the B-cell surface, distinct from that of other anti-CD20 antibodies, and induces potent B-cell lysis and depletion. The selective mechanism of action and subcutaneous administration of ofatumumab allows precise delivery to the lymph nodes, where B-cell depletion in MS is needed, while sparing those in the spleen that help maintain protective immunity. Once-monthly dosing of ofatumumab also allows faster repletion of B-cells, and offers more flexibility as no first dose observations or laboratory monitoring is required. Novartis obtained rights for ofatumumab from Genmab in all indications, including MS, in December 2015.
Novartis (BAF312)
Siponimod is an investigational, selective modulator of specific subtypes of the sphingosine-1-phosphate (S1P) receptor. Siponimod binds to the S1P1 sub-receptor on lymphocytes, which prevents them from entering the central nervous system (CNS) of patients with multiple sclerosis. This leads to the anti-inflammatory effects of siponimod. Siponimod also enters the CNS and binds to the S1P5 sub-receptor on specific cells in the CNS (oligodendrocytes and astrocytes). By binding to these specific receptors, siponimod has the potential to modulate damaging cell activity, and preclinical studies suggest that it may prevent synaptic neurodegeneration and promote remyelination in the central nervous system.
Sanofi (Teriflunomide)
Teriflunomide is the first original ‘once-daily’ oral ‘disease modifying therapy’ (DMT) for Multiple Sclerosis. It offers an effective, safe and a convenient option that is indicated as a first-line treatment for relapsing forms of Multiple Sclerosis that must be taken once a day, with or without food.
Emerging Pipeline Landscape of Multiple Sclerosis (MS)
| Company name | Product Name | Stage of development | Route of administration | Mechanism of action |
| Merck Healthcare KGaA | Evobrutinib + Teriflunomide | Phase III | Oral | BTK inhibtor |
| Actelion | Ponesimod | Phase III | Oral | S1P1 immunomodulator |
| Novartis | BAF312 (Mayzent™) | Phase III | Oral | S1P1 Modulator |
| Novartis | Ofatumumab | Phase III | Subcutaneous | anti-CD20 |
| MedDay Pharmaceuticals | MD1003 | Phase III | Oral | Enzyme modulators |
| F. Hoffmann-La Roche AG | Ocrelizumab/ RG1594 | Phase III | Intavenous | anti- CD20 positive B- cells |
| Sanofi | Teriflunomide | Phase III | Oral | Dihydroorotate dehydrogenase inhibitors |
| Sanofi | Alemtuzumab | Phase III | Intravenous | dihydro-orotate dehydrogenase inhibitor |
| TG Therapeutics | Ublituximab | Phase III | Oral | anti- CD20 |
| Biogen | Opicinumab | Phase II | Intravenous | anti-LINGO |