Over the last few days, the coronavirus outbreak has spread to other countries such as China, South Korea, Italy, Iran, Japan, Singapore, Hong Kong, the United States, and other countries. It has endangered thousands of lives in a short span of time. Hence, several pharmaceutical & Biotech companies are focusing on the development of novel therapies for the treatment of coronavirus.
Coronavirus outbreak begins to boosting the development of new treatment. The pipeline landscape of the coronavirus is robust with the involvement of several therapies such as vaccines, antibiotics, anti-viral therapies, mesenchymal stem cell (MSC) derived and other therapies for the treatment of coronavirus.
Several pharmaceutical & Biotech companies have entered into the collaboration with the National Institutes of Health (NIH), for the development of novel coronavirus therapies.
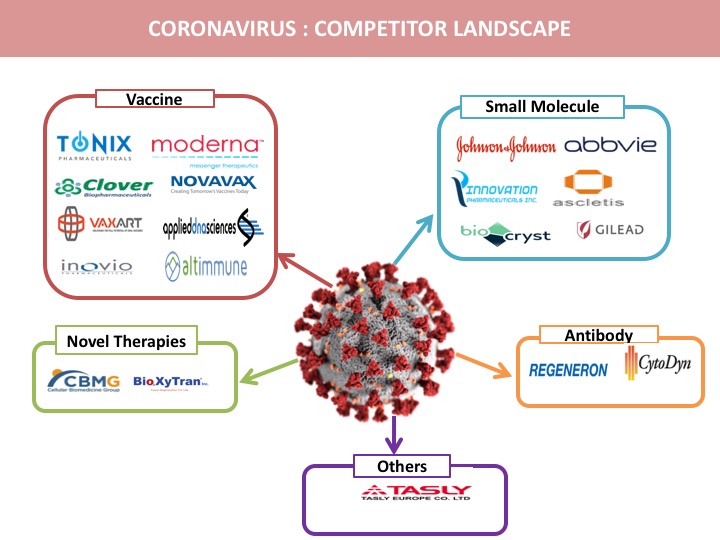
Pipeline Therapies for the treatment of Coronavirus
Vaccine
Pharmaceutical companies are developing coronavirus vaccines that have the potential to become a substantial treatment for the contagious coronavirus infection across the world. There are major drugs in the pipeline that have the potential to address the Wuhan outbreak as follows
TNX-1800 (Tonix Pharmaceuticals)
Tonix Pharmaceuticals has entered into the collaboration with Southern Research to develop a Potential Vaccine to Protect against New Coronavirus Disease 2019 (COVID-19) based on HorsepoxVirus (TNX-1800) using Tonix’s proprietary Horsepox Vaccine Platform. Horsepox is closely related to vaccinia vaccines, which are a group of orthopoxviruses that have been used as smallpox vaccines. TNX-1800 is designed to express a protein derived from the virus that causes the coronavirus infection. Under the terms of the agreement, Southern Research will be responsible for evaluating the efficacy of the vaccine.
INO-4800 (Inovio Pharmaceuticals and Beijing Advaccine Biotechnology)
Inovio Pharmaceuticals has partnered with Beijing Advaccine Biotechnology Company for the development of INO-4800, a novel coronavirus vaccine through Phase I human testing in the U.S. to evaluate safety and immunogenicity. The Company has begun testing and clinical product. Preclinical manufacturing preparations are underway. The company has received the grant up to $9 million from the Coalition for Epidemic Preparedness Innovations (CEPI) for initiating the testing of INO-4800.
Collaboration with Beijing Advaccine Biotechnology Company would enable the Inovio Pharmaceuticals to rapidly develop its new vaccine by running parallel Phase I trials in China.
Recombinant Subunit Vaccine (Clover Biopharmaceuticals)
Clover Biopharmaceuticals is developing a recombinant subunit vaccine using its patented Trimer-Tag technology. The company is developing the vaccine based on the trimeric S protein (S-Trimer) of the 2019-nCoV virus, which is responsible for binding with the host cell and causing a viral infection.
Using this technology, Clover successfully produced the subunit vaccine in a mammalian cell-culture based expression system. The company also identified antigen-specific antibodies in the serum of fully recovered patients who were previously infected by the virus. A highly purified form of the S-Trimer vaccine is expected to be available in six to eight weeks for performing pre-clinical studies. The company is equipped with in-house cGMP biomanufacturing capabilities to scale-up production if the vaccine is proven to be successful.
Clover has entered into the collaboration with collaborated with GlaxoSmithKline plc. Under this research collaboration, the companies would evaluate Clover’s protein-based coronavirus vaccine candidate (COVID-19 S-Trimer) with GSK’s pandemic adjuvant system. GSK’s pandemic adjuvant system would help further assess COVID-19 S-Trimer in preclinical studies.
According to GSK Vaccines Chief Medical Officer, “We are proud to contribute to cutting edge research from scientists at Clover Biopharmaceuticals in China as part of our strategy to make our adjuvant technology available to selected partners who have a promising vaccine candidate against the newly emerged coronavirus.”
Linear DNA Vaccine (Applied DNA Sciences and Takis Biotech)
Applied DNA Sciences Inc., has expanded its existing Joint Development Agreement (JDA) with Takis Biotech for the preclinical development of a linear DNA vaccine against 2019-nCoV, the new coronavirus.
The PCR-produced linear DNA would provide advantages such as high purity, increased production speed, and absence of antibiotics and bacterial contaminants. Moreover, the vaccine is effective to be inserted into the patient’s genome.
MERS Coronavirus Vaccine (Novavax)
A novel Middle East Respiratory Syndrome (MERS) coronavirus vaccine is under development by Novavax. It acts by binding to the major surface S-protein and developed using the company’s recombinant nanoparticle vaccine technology.
It is a crucial target for coronavirus vaccine development by the Coalition for Epidemic Preparedness Innovations (CEPI) and is a priority disease for the World Health Organisation (WHO). The company plans to conduct the human trials in 2020 and Phase I clinical trial planned in late spring. It is tested along with the Novavax’s proprietary adjuvant Matrix-M, it inhibited infection by inducing immune responses in the laboratory studies.
INO-4700 (Inovio Pharma)
InovioPharma in partnership with GeneOne Life Science has developed, INO-4700 (GLS-5300) the investigational DNA immunotherapy Drug. It is delivered as intramuscularly, using the Cellectra delivery device. The vaccine was well-tolerated and demonstrated high immune responses against the MERS-CoV in 94% of patients in the early-stage clinical trial in July 2019. It has generated broad-based T cell responses in 88% of the subjects.
mRNA-1273 (Moderna)
Moderna has released the first batch of its vaccine i.e. mRNA-1273 for human use. mRNA-1273 is an mRNA vaccine against the novel coronavirus encoding for a prefusion stabilized form of the Spike (S) protein complex is necessary for membrane fusion and host cell infection and has been the target of vaccines against the coronaviruses responsible for Middle Eastern Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), which was selected by Moderna in collaboration with investigators at the NIAID Vaccine Research Center (VRC).
National Institute of Allergy and Infectious Diseases (NIAID) and National Institutes of Health (NIH) is planning to conduct the phase I study of mRNA-1273 in the United States. The manufacture of this batch was funded by the Coalition for Epidemic Preparedness Innovations (CEPI).
Small Molecule
The article, “Therapeutic options for the 2019 novel coronavirus (2019-nCoV) “, published by the British magazine, Nature Reviews Drug Discovery, on February 10, 2020, suggests that existing anti-HIV and anti-HCV drugs may have inhibitory effects against novel coronavirus.
Brilacidin (Innovation Pharmaceuticals)
Innovation Pharmaceuticals signed a Material Transfer Agreement (MTA) with one of the country’s 12 Regional Biocontainment Labs (RBLs) to research its lead defensin mimetic drug candidate, Brilacidin, as a potential novel coronavirus treatment. Brilacidin has demonstrated antibacterial, anti-inflammatory and immunomodulatory properties in viral infections, including inhibition of SARS-CoV-2, the virus responsible for COVID-19. It is also used in several other clinical trials. The Company is planning to explore research collaborations and seek federal grants to develop the coronavirus drug. It is already investigating the drug for inflammatory bowel disease and oral mucositis in cancer patients.
Remdesivir (GS-5734) (Gilead Sciences in collaboration with NIH)
National Institute of Health has begun the randomized, controlled clinical trial for evaluating the safety and efficacy of antiviral remdesivir among hospitalized adults diagnosed with coronavirus disease 2019 (COVID-19).
The trial has been initiated at the University of Nebraska Medical Center (UNMC) in Omaha. The first trial participant is an American who was repatriated after being quarantined on the Diamond Princess cruise ship that docked in Yokohama, Japan and volunteered to participate in the study.
According to The New England Journal of Medicine (NEJM), the administration of remdesivir improved the clinical condition. GlaxoSmithKline had entered into the collaboration with Gilead to develop an effective treatment against the coronavirus as confirmed cases of the novel coronavirus are rising.
Galidesivir (Biocryst Pharma in collaboration with NIH)
BioCryst is developing galidesivir in collaboration with the US Government Agencies and other institutions. The antiviral drug Galidesivir (BCX4430) has shown broad-spectrum activity against a wide range of pathogens including coronavirus. It is a nucleoside RNA polymerase inhibitor that disrupts the process of viral replication. A Phase I clinical safety and pharmacokinetic study in healthy subjects has been completed and in animal studies.
Galidesivir has also demonstrated broad-spectrum activity in vitro against more than 20 ribonucleic acids (RNA) viruses in nine different families, including filoviruses, togaviruses, bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses, and flaviviruses.
Lopinavir (AbbVie)
AbbVie’s Lopinavir HIV protease inhibitor is being studied along with ritonavir for the treatment of MERS-CoV and SARS-CoV. This combination therapy is believed to act on the intracellular processes of coronavirus replication.
Darunavir/Cobicistat (Johnson & Johnson)
Pharmaceutical giant Johnson & Johnson is working to develop a vaccine for the emergent coronavirus identified last month in Wuhan, China, launching initial efforts to construct from the virus’ genetic sequence a candidate that could be tested in humans.
Janssen Pharmaceutical (a subsidiary of Johnson & Johnson) donated its PREZCOBIX HIV medication (darunavir/cobicistat) for use in research activities aimed at finding a treatment for Covid-19.
Ascletis Pharmaceuticals Co., Ltd.
Hangzhou-based Ascletis Pharma applied to the Chinese authorities to test two HIV protease inhibitors (ritonavir and ASC09) in clinical trials to treat COVID-19. Another combination therapy, Ganovo (danoprevir) and Ritonavir, is in development by Ascletis Pharma in collaboration with Ninth Hospital of Nanchan.
Antibody
Leronlimab (CytoDyn)
CytoDyn is examining leronlimab (PRO 140), a CCR5 antagonist, as a potential coronavirus drug. The drug is already being investigated in Phase II clinical trials for treatment for HIV and has been awarded fast-track approval status by the Food and Drug Administration (USFDA).
REGN3048-3051 (Regeneron in collaboration with NIH)
Discovered by Regeneron, the combination of neutralizing monoclonal antibodies REGN3048 and REGN3051 is being studied against coronavirus infection in a first-in-human clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). The safety and tolerability of the drug will be studied in 48 patients.
Novel Therapies
MSCs-derived exosomes (Cellular Biomedicine Group)
Cellular Biomedicine Group conducted a Phase I clinical trial (NCT04276987) for MSC –derived exosomes to explore the safety and efficiency of aerosol inhalation of the exosomes derived from allogeneic adipose mesenchymal stem cells (MSCs-Exo) in severe patients with novel coronavirus pneumonia (NCP).
BXT-25 (Bioxytran)
US-based biotechnology company Bioxytran has announced plans to develop a peptide-based therapy, BXT-25, to treat late-stage patients infected with the new coronavirus who have acute respiratory distress syndrome (ARDS). The diffusion of oxygen to the blood is comprised of patients suffering from ARDS leading to fluid build-up in the lungs.
It is designed to be 5,000 times smaller than blood cells and efficiently transport oxygen through the body for a period of nine hours before being processed by the liver. The drug helps in supplying oxygen to the vital organs and enables the patient to recover and survive.
Other
T89 (Tasly Pharmaceuticals, Inc.)
Tasly Pharmaceuticals has conducted an open-label, randomized clinical study to investigate the effect of T89 on improving oxygen saturation and clinical symptoms in patients with Coronavirus Disease 2019 (COVID-19).
Xiyanpinginjection (Jiangxi Qingfeng Pharmaceutical Co. Ltd)
Xiyanping is primarily composed of 9-dehydro-17-hydro-andrographolide and sodium 9-dehydro-17-hydro-andrographolide-19-yl sulfate. Xiyanping is an anti-inflammatory and antiviral preparation developed and licensed for use in China. It is a semi-synthetic injectable product derived from the active component of the plant Andrographispaniculata, which is used in Traditional Chinese medicine. Qingfeng Pharmaceutical is conducting a randomized, open-label trial to evaluate the safety and efficacy of Xiyanping injection in patients with 2019-nCoV pneumonia.
Thanks for the blog article.Much thanks again. Much obliged.