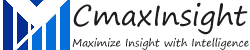Izervay (avacincaptad pegol sodium)
FDA approved Izervay, a complement C5 inhibitor, for the treatment of geographic atrophy secondary to age-related macular degeneration, according to a press release from Astellas Pharma.
The approval was based on the data from the GATHER1 and GATHER2 phase 3 clinical trials in which monthly 2 mg injections of Izervay (avacincaptad pegol intravitreal solution) showed a statistically significant reduction (P < .01) in the rate of geographic atrophy growth at 12 months. The slowing in disease progression was seen as early as 6 months, while up to a 35% reduction in progression occurred in the first year of treatment.
Talvey (talquetamab-tgvs)
FDA granted accelerated approval of Talvey (talquetamab-tgvs), a first-in-class bispecific antibody for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody.
Talvey is a bispecific T-cell engaging antibody that binds to the CD3 receptor on the surface of T cells and G protein-coupled receptor class C group 5 member D (GPRC5D) expressed on the surface of multiple myeloma cells, non-malignant plasma cells and healthy tissue such as epithelial cells in keratinized tissues of the skin and tongue.
Elrexfio (elranatamab-bcmm)
FDA granted accelerated approval to elranatamab-bcmm (Elrexfio, Pfizer, Inc.), a bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager, for adults with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
Sohonos (palovarotene)
FDA approved Sohonos (palovarotene) capsules as a retinoid indicated for the reduction in volume of new heterotopic ossification in adults and pediatric patients aged 8 years and older for females and 10 years and older for males with fibrodysplasia ossificans progressiva (FOP).
The FDA approval was based on the pivotal efficacy and safety data from the Phase 3 MOVE trial, the first and largest multicenter, open-label trial in adult and pediatric patients.
Veopoz (pozelimab-bbfg)
FDA approved Veopoz (pozelimab-bbfg) for the treatment of adult and pediatric patients 1 year of age and older with CHAPLE disease, also known as CD55-deficient protein-losing enteropathy. Veopoz is the first and only treatment indicated specifically for CHAPLE. Veopoz, a fully human monoclonal antibody, is designed to target complement factor C5, a protein involved in complement system activation.
The FDA approval was based on results from a Phase 2/3 open-label trial that investigated the efficacy and safety of pozelimab in 10 patients aged 3 to 19 (median of 8.5 years).
Eylea HD (aflibercept)
FDA approved EYLEA HD (aflibercept) Injection 8 mg for the treatment of patients with wet age-related macular degeneration (wAMD), diabetic macular edema (DME) and diabetic retinopathy (DR). This is the first and only treatment approved in wAMD and DME for immediate dosing at 8-week and up to 16-week intervals following three initial monthly doses
Approval was based on the pivotal PULSAR and PHOTON trials in which EYLEA HD demonstrated clinically equivalent vision gains to EYLEA (aflibercept) Injection 2 mg that were maintained with fewer injections.
Tyruko (natalizumab-sztn)
FDA approved biosimilar Tyruko (natalizumab-sztn), developed by Polpharma Biologics. Tyruko is approved to treat all indications covered by the reference medicine and is the first and only FDA-approved biosimilar for relapsing forms of multiple sclerosis (MS).
Sandoz entered into a global commercialization agreement for Tyruko with Polpharma Biologics in 2019. Under this agreement, Polpharma Biologics will maintain responsibility for development, manufacturing and supply of the active substance in Tyruko. Through an exclusive global license, Sandoz has the rights to commercialize and distribute it in all markets.