Regulatory T cells (Tregs) are a specialized CD4+ subpopulation of lymphocytes with regulatory functions that suppress excessive and uncontrolled immune responses, which inhibit adaptive and innate immune cells such as conventional T cells, B cells, antigen-presenting cells (APCs), natural killer (NK) cells, and so forth.
The most specific marker for these cells is FoxP3, which is localized intracellularly. Selected surface markers such as CD25high (high molecular density) and CD127low (low molecular density) could serve as surrogate markers to detect Tregs in routine clinical practice. Dysregulation in Treg cell frequency or functions may lead to the development of autoimmune disease.
Regulatory T (Treg) cells maintain immune homeostasis by inhibiting abnormal/overactive immune responses to both autogenic and nonautogenic antigens. Treg cells play an important role in immune tolerance, autoimmune diseases, infectious diseases, organ transplantation, and tumor diseases.
- Organ transplantation is the gold standard therapy for end-stage organ failure. Although, the results of organ transplantation have been ameliorated in recent decades, chronic rejection and the side effects of immunosuppressants are still an ongoing serious issue. None of the present immunosuppressive medications (in contrast to Tregs) have the potential to specifically suppress immune mechanisms. Various strategies are currently underway to avoid or minimize the use of immunosuppressive drugs. In this case, it may be possible that Tregs represent a promising solution to induce transplantation tolerance and control the immune response.
- Autoimmune diseases as lifelong disorders are one of the major causes of mortality. The main etiology of autoimmune diseases is not fully understood; however, failure of immunological tolerance is a common cause of each autoimmune condition.
- Regulatory T (Treg) cells, possess a strategic role in the maintenance of immune homeostasis, and their function has been closely linked to the development of diverse pathologies including autoimmunity and cancer. Consistently recent studies have highlighted that Treg dysfunction is a common denominator in autoimmunity, with reduced Treg cell frequencies and impaired suppressive function identified in a wide range of autoimmune diseases, including multiple sclerosis, SLE, type 1 diabetes, thyroiditis, and inflammatory bowel disease. Thus, it is becoming apparent that Treg cells possess a unique power in supervising autoimmune reactions and the re-establishment of self-tolerance.
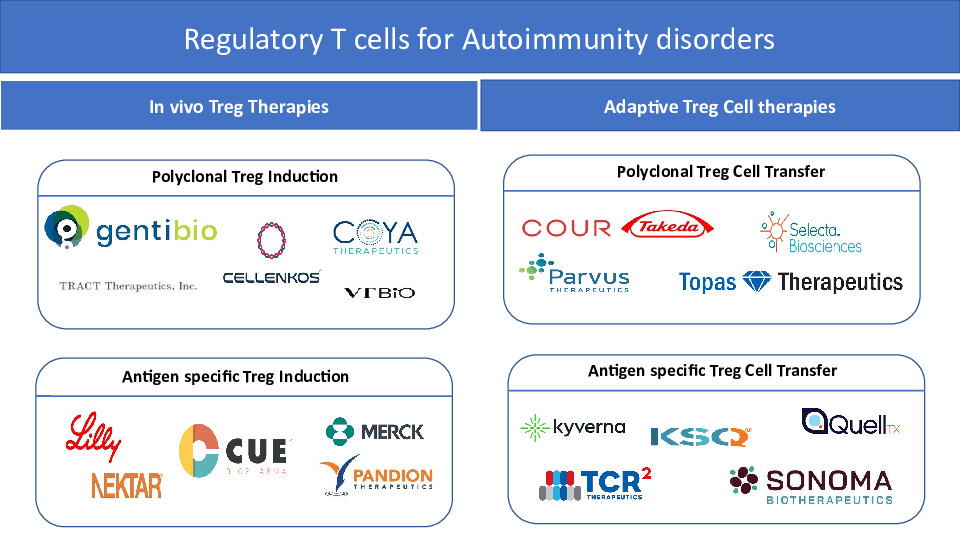
- Two main strategies have been developed to augment the numbers and/or increase the functional activity of Tregs. In vivo Treg therapies focus on the administration of immunomodulatory agents that enhance the number and/or function of Treg cells in vivo, and the adoptive Treg therapies transfer in vitro expanded Treg cells. Interventions that increase polyclonal endogenous Treg cells in vivo involve low-dose interleukin-2 (IL-2), mutant IL-2, IL2/Anti-IL-2 Ab complexes. In contrast, applications of antigen-based treatments could lead to the enhancement of antigen-specific Treg subsets. On the other hand, adoptive Treg cell therapies rely on the optimal isolation and expansion of Treg cells in vitro. Thus far, clinical trials in autoimmunity have only utilized expanded polyclonal Treg cell populations. However, antigen-specific Treg cells can be generated in vitro by genetic insertion of synthetic receptors (including engineered T cell receptors (TCR), chimeric antigen receptors (CAR) or B cell antibody receptors (BAR)), or by transformation of antigen-specific effector T (Teff) cells into induced Treg (iTreg) cells via stimulation in the presence of transforming growth factor beta (TGF-ß) and IL-2, transgenic FOXP3 overexpression, blockade of cyclin-dependent kinase 8 (CDK8) and CDK19 signaling, or a combination of cytotoxic T lymphocyte antigen 4 (CTLA-4) overexpression, IL-2 ablation and antigenic stimulation.
- Therapeutical Treg modulation is considered to be a promising therapeutical approach to treat some selected disorders, such as allergies, autoimmune disorders, and to prevent allograft rejection. Multiple approaches such as adoptive Treg cell therapies as well as targeting mediators that can enhance the action of endogenous Treg cells are under evaluation, both in academia and industry.