|
SPONSOR
|
PRODUCT
|
DESCRIPTION
|
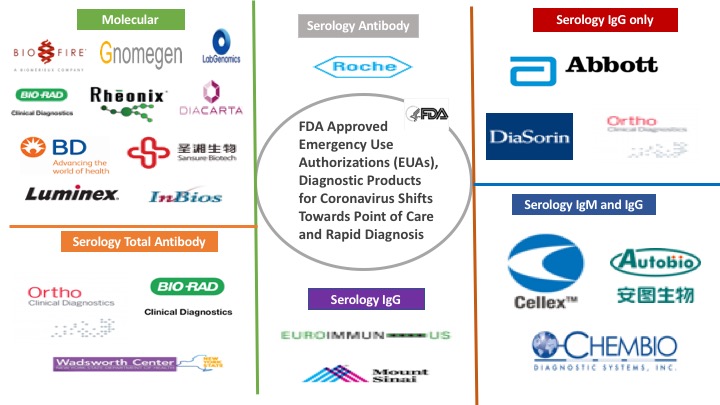
| Center for Disease Control and Prevention (CDC) | CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel | Developed by CDC and initially distributed to public health labs across the country
Can only be run in high complexity labs |
| Wadsworth – New York State Public Health | New York SARS-CoV-2 Real-time Reverse Transcriptase (RT)-PCR Diagnostic Panel | Developed by Wadsworth based on CDC’s published protocol Run in qualified labs across New York State Can only be run in high complexity labs |
|
Roche Molecular Systems, Inc.
| cobas SARS-CoV-2 for use on the cobas 6800/8800 Systems | Commercially distributed as a kit to labs
Can be run in moderate and high complexity labs |
|
Life Technologies (a part of
Thermo Fisher Scientific, Inc.)
| TaqPath COVID-19 Combo Kit, 100 Rxn, TaqPath COVID19 Combo Kit, 1,000 Rxn | Commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Laboratory Corporation of America | COVID-19 RT-PCR Test COVID-19 RT-PCR Amendment | Developed and run in high complexity LabCorp labs only; not for broader lab distribution
Amendment permits use of the Pixel by LabCorp COVID-19 test home collection kit allowing patients to self-collect nasal swab specimens at home
The kit provides specimen collection materials and materials to safely mail specimens to an authorized laboratory |
| Hologic, Inc. | Panther Fusion SARS-CoV-2 Assay | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Quest Diagnostics Infectious Disease, Inc. | SARS-CoV-2 RNA, Qualitative Real-Time RT-PCR | Developed and run in Quest labs only; not a kit for distribution
Can only be run in high complexity labs |
| Quidel Corporation | Lyra SARS-CoV-2 Assay | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Abbott Molecular, Inc. | Abbott RealTime SARS-CoV-2 assay | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| GenMark Diagnostics, Inc. | ePlex SARS-CoV-2 Test | Reagents commercially distributed as a kit to labs
Can run up to 24 specimens at the same time
Can be run in a moderate or high complexity lab |
| DiaSorin Molecular LLC | Simplexa COVID-19 Direct | Reagents commercially distributed as a kit to labs
Can run 1 specimen at a time
Can be run in moderate and high complexity labs |
| Primerdesign Ltd. | Primerdesign Ltd COVID-19 genesig Real-Time PCR | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Cepheid | Xpert Xpress SARS-CoV-2 test | Reagents commercially distributed as a kit to labs
Can run up to 2,000 samples per day Can be run in a high or moderate complexity lab or at the Point of Care (POC) near the patient (deemed CLIA waived) |
| Mesa Biotech Inc | Accula SARS-CoV-2 Test | Reagents commercially distributed as a kit to labs
Runs one specimen at a time
Can be run in a high or moderate complexity lab or at the Point of Care (POC) near the patient (deemed CLIA waived) |
| BioFire Defense, LLC | BioFire COVID-19 Test | Reagents commercially distributed as a kit to labs
Can run up to 264 tests per day
Can be run in moderate or high complexity labs |
| PerkinElmer, Inc. | PerkinElmer New Coronavirus Nucleic Acid Detection kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Avellino Labs USA | AvellinoCoV2 test | Developed and run in Avellino labs; not distributed to other labs
High complexity test limited to authorized laboratories |
| BGI Genomics Co. Ltd. | Real-Time Fluorescent RT-PCR Kit for Detecting SARS-2019- nCoV | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Luminex Molecular Diagnostics, Inc. | NxTAG CoV Extended Panel Assay | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Abbott Diagnostics Scarborough, Inc. | ID NOW COVID-19 | Reagents commercially distributed as a kit Requires a specific platform (ID NOW), of which there are 18,000 installed across the US
Runs one specimen at a time |
| NeuMoDx Molecular, Inc | NeuMoDx SARS-CoV-2 Assay | Reagents commercially distributed as a kit
Can run 288 or 96 samples at once, depending on the instrument, and takes 80 minutes per sample
Can be run in high and moderate complexity labs |
| QIAGEN GmbH | QIAstat-Dx Respiratory SARSCoV-2 Panel | Detects multiple other respiratory viral and bacterial organisms
Reagents commercially distributed as a kit to labs
Runs one specimen at a time and takes one hour
Can be run in high and moderate complexity labs |
|
EUA for COVID-19 LDTs
| Laboratory developed tests that are authorized are listed below and hyper link to letter granting inclusion under EUA | Authorizes the use of LDTs that meet certain criteria
Authorized tests can be used in the high complexity CLIA-certified lab that developed the test |
| Ipsum Diagnostics | COV-19 IDx Assay | Uses commercially available reagents
Can only be run in high complexity labs by Ipsum |
| Becton, Dickinson & Company (BD) | BioGX SARS-CoV-2 Reagents for BD MAX System | Reagents commercially distributed as a kit to labs
Fully automated, 8 samples per hour Can be run in moderate and high complexity labs |
|
Luminex Corporation
| ARIES SARS-CoV-2 Assay | Reagents commercially distributed as a kit to labs
Can be run in moderate and high complexity labs |
|
ScienCell Research Laboratories
| ScienCell SARS-CoV-2 Coronavirus Real-time RT-PCR (RT-qPCR) Detection Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| CoDiagnostics, Inc. | Logix Smart Coronavirus Disease 2019 (COVID-19) kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Gnomegen LLC | Gnomegen COVID-19 RT-Digital PCR Detection Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| InBios International, Inc | Smart Detect SARS-CoV-2 rRTPCR Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| DiaCarta, Inc. | QuantiVirus SARS-COV-2 Test Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Becton, Dickinson & Company (BD) | BD SARS-CoV Reagents for BD MAX System | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Atila BioSystems, Inc. | iAMP COVID-19 Detection Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Maccura Biotechnology (USA) LLC | SARS-CoV-2 Fluorescent PCR Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
|
GenoSensor, LLC.
| GS COVID-19 RT-PCR KIT | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
|
KorvaLabs Inc.
| Curative-Korva SARS-Cov-2 Assay | Laboratory Developed Test
High complexity test limited to KorvaLabs, Inc., a certified high complexity laboratory |
| Fosum Pharma USA Inc | Fosun COVID-19 RT-PCR Detection Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| OSANG Healthcare | GeneFinder COVID-19 Plus RealAmp Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Trax Management Services Inc. | PhoenixDx 2019-CoV | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Seegene, Inc. | Allplex 2019-nCoV Assay | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Altona Diagnostics GmbH | RealStar SARS-CoV02 RT-PCR Kits U.S. | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| SD Biosensor, Inc. | STANDARD M nCoV RealTime Detection Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| SEASUN BIOMATERIALS | U-TOP COVID-19 Detection Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Rheonix, Inc. | Rheonix COVID-19 MDxAssay | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
|
LabGenomics Co., Ltd.
| LabGunCOVID-19 RT-PCR Kit | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| Bio-Rad Laboratories, Inc. | Bio-Rad SARS-CoV-2 ddPCR Test | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |
| BioFire Diagnostics, LLC | BioFire Respiratory Panel 2.1 (RP2.1) | Reagents commercially distributed as a kit to labs
Can only be run in high complexity labs |