Cystic Fibrosis is an autosomal, recessive inheritable genetic disease that causes severe damage to the respiratory and digestive systems. This damage often results from a buildup of thick, sticky mucus in the organs. It occurs as a result of a defect in what’s called the “cystic fibrosis transmembrane conductance regulator” gene, or CFTR gene. This gene controls the movement of water and salt in and out of your body’s cells. A sudden mutation causes the abnormality in CFTR gene and commonly affected the various organs including the lungs, pancreas, liver, and intestines. The symptoms of cystic fibrosis can vary depending on the person and the severity of the condition. The age at which symptoms develop can also differ. Symptoms may appear at infancy, but for other children, symptoms may not begin until after puberty or even later in life. As time passes, the symptoms associated with the disease may get better or worse.
Around 70,000-80,000 people worldwide are suffering from the Cystic Fibrosis. Cystic Fibrosis has the highest incidence and prevalence in Caucasian populations and is less common in other population groups. Every year, around 1,000 people are diagnosed with Cystic Fibrosis in the United States.
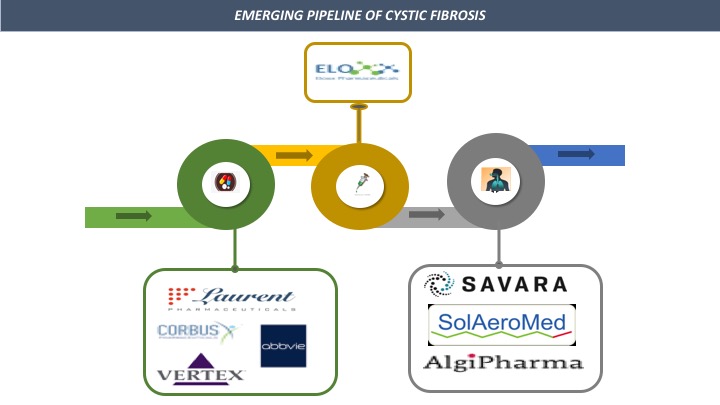
Several pharmaceutical companies are developing the novel drugs for the treatment of Cystic Fibrosis. The pipeline landscape of the cystic fibrosis is robust with the involvement of several drugs such as vancomycin hydrochloride inhalation powder, OligoG, ELX-02 and VX-561 etc.
The pharmaceutical companies involved in developing emerging drugs against Cystic Fibrosis are as follows:
Savara Pharmaceuticals (AeroVanc)
AeroVanc (vancomycin hydrochloride inhalation powder) is an inhaled dry powder form of vancomycin for the treatment of the persistent (MRSA) lung infection in people living with Cystic Fibrosis. AeroVanc is in Phase III stage of development for the treatment of the Methicillin-resistant Staphylococcus aureus (MRSA) lung infection in Cystic Fibrosis. AeroVanc is effective in improving the clinical efficacy, and reducing the adverse effects due to lessor systemic drug exposure in comparison to the intravenous antibiotic treatment.
AlgiPharma (OligoG)
OligoG is a dry powder drug that has been shown to decrease the thickness of mucus in the lungs and may help individuals with cystic fibrosis clear mucus easier. OligoG help in improving the effectiveness of some antibiotics. It is administered using a dry powder inhaler and also developed as a liquid for use with a nebulizer
Currently, Phase IIb trial is currently running in Australia, with another Phase 2b scheduled to start in Europe H1 2020. The drug candidate OligoG for Cystic Fibrosis has Orphan Drug designation from both the European Medicines Agency and the FDA
Eloxx Pharmaceuticals, Inc. (ELX-02)
ELX-02, is a small molecule drug candidate designed to restore production of full-length functional proteins. It is in the early stages of clinical development focusing on cystic fibrosis and nephropathic cystinosis. ELX-02 is an investigational drug that has not been approved by any global regulatory body.
Furthermore, ELX-02, is a eukaryotic ribosomal selective glycoside (ERSG) designed to increase the read-through activity in patients with nonsense mutations and enable the production of sufficient amounts of full-length functional protein to restore activity.
Its Phase II program has been given a score of “high priority” by the European Cystic Fibrosis Society-Clinical Trial Network (ECFS-CTN).
Laurent Pharmaceuticals Inc. (LAU-7b)
LAU-7b is a novel and improved solid dosage form of fenretinide, requiring. once-a-day oral administration. It was recently tested in adult patients with Cystic Fibrosis in a dose-ascending where Phase Ib study, showing a good safety and tolerability, and promising pharmacokinetic and pharmacodynamic results.
The company is currently recruiting patients for its Phase II safety and efficacy study in adult patients with Cystic Fibrosis. The goal of the Phase II trial is to evaluate LAU-7b’s effect on the preservation of lung function, by reducing persistent unresolved inflammation in the lung and stimulating its return to homeostasis. The study would enroll 136 adults with Cystic Fibrosis for a treatment duration of 6 months and will involve more than 30 clinical sites in the USA and Canada.
Corbus Pharmaceuticals Inc. (Lenabasum)
Lenabasum is a rationally-designed, oral, small-molecule that selectively binds as an agonist to the cannabinoid receptor type 2 (CB2). It has a direct effect on fibroblasts to limit the production of fibrogenic growth factors and extracellular connective tissue that lead to tissue fibrosis. Lenabasum treatment has resulted in lowering the and longer time to pulmonary exacerbations in Phase II cystic fibrosis study. The Lenabasum had demonstrated the safety and tolerability profile in clinical studies. It has demonstrated safety and tolerability profile in clinical studies
Vertex Pharmaceuticals Incorporated (VX-561)
VX-561 is a deuterated form of ivacaftor that replaces one or more hydrogen atoms with deuterium. It is designed to keep CFTR proteins at the cell surface open longer to improve the flow of salt and water across the cell membrane, which helps hydrate and clear mucus from the airways.
Vertex Pharmaceuticals Incorporated (VX-121 + TEZACAFTOR + VX-561)
VX-121 and tezacaftor act by increasing the amount of mature protein at the cell surface by targeting the processing and trafficking defect of the CFTR protein. VX-561 (deuterated ivacaftor) is designed to keep CFTR proteins at the cell surface open longer to improve the flow of salt and water across the cell membrane, which helps hydrate and clear mucus from the airways.
SolAeroMed Inc (S-1226)
S-1226 is a novel fast-acting bronchodilator, with airway lubricant properties to remove mucus. It acts as a cough suppressant and is an anti-inflammatory agent. S-1226 is based on amalgation of Perflubron (a synthetic surfactant derivative) and carbon dioxide. When combined in inhalation therapy as S-1226 delivered by nebulization these compounds offer significant promise to treat respiratory diseases where breathing is compromised due to bronchoconstriction, excess mucus production, and/or impaired mucus motility. It has demonstrated the safety and efficacy for the treatment of Cystic Fibrosis.
Cystic Fibrosis Emerging Pipeline Landscape
| Company Name | Product name | Stage of development | Mechanism of Action | Route of administration |
| Savara Inc. | Vancomycin inhalation powder | Phase III | Cell wall inhibitors | Inhalation |
| AlgiPharma AS | OligoG DPI | Phase II | Bacterial growth inhibitors | Inhalation |
| Eloxx Pharmaceuticals, Inc. | ELX-02 | Phase II | Ribosomal protein modulators | Subcutaneous |
| Corbus Pharmaceuticals Inc. | Lenabasum | Phase II | Cannabinoid receptor CB2 agonists | Oral |
| Laurent Pharmaceuticals Inc. | LAU-7b | Phase II | Retinoic acid receptor agonists | Oral |
| Vertex Pharmaceuticals Incorporated | VX-121 + TEZACAFTOR + VX-561 | Phase II | Regulated CFTR gene | Oral |
| SolAeroMed Inc. | S-1226 | Phase II | Cell membrane permeability enhancers | Inhalation |
| Vertex Pharmaceuticals Incorporated | VX-561 | Phase II | Regulated CFTR gene | Oral |