Vitiligo is a chronic disorder that causes areas of skin to lose color. When skin cells that make color are attacked and destroyed, the skin turns a milky-white color. Various factors such as autoimmune disease, genetic factors, & neurogenic factors lead to the development of Vitiligo. The main symptom associated with vitiligo is the loss of natural color. The patches can show up on any part of your body and can affect the skin, which gets milky-white patches, usually on the hands, feet, arms, and face, hair, which can turn white on the scalp, eyebrow, eyelash, and beard & the inside of your mouth or nose. People with vitiligo can also get problems with the eyes and ears. In addition, people with the disorder may worry about how their skin looks, which can affect general well-being.
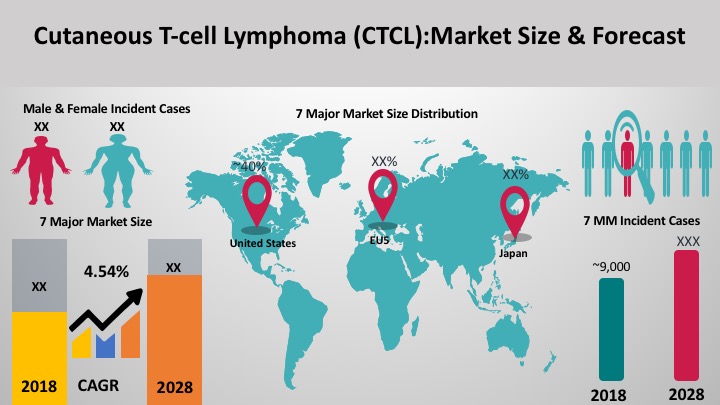
Vitiligo is the most common skin depigmentation disorder with an average prevalence estimated to be between 0.5% to 2% of the global population. An estimated 65-95 million people of all ages, sexes, and ethnicities worldwide suffer from vitiligo, including approximately 2.4 million people in the United States. The majority (70–80%) of patients with vitiligo experience disease onset before age 30. Approximately 30% have childhood-onset vitiligo before age 12.
Currently, there are no FDA-approved therapies for vitiligo. Dermatologists typically prescribe topical corticosteroids, topical calcineurin inhibitors, and/or phototherapy for the treatment of vitiligo. Therefore, pharmaceutical companies such as Incyte Corporation, Dermavant Sciences GmbH, Aclaris Therapeutics, Inc. Amgen and other companies are developing novel therapies for the treatment of Vitiligo.
Incyte Corporation (Ruxolitinib/INCB018424)
Ruxolitinib is a Janus kinase (JAK1/JAK2) inhibitor discovered by Incyte scientists. Overactive signaling through the JAK-STAT pathway has been associated with many types of disease, including a group of rare blood cancers called myeloproliferative neoplasms (MPNs), as well also used for the treatment of Vitiligo. Ruxolitinib is currently under the clinical trial of Phase III. The purpose of this study is to evaluate the efficacy and safety of ruxolitinib cream in adolescent and adult participants with non-segmental vitiligo for whom total body involved vitiligo area (facial and nonfacial) does not exceed 10% body surface area (BSA).
Dermavant Sciences GmbH (Cerdulatinib 0.37% gel/DMVT-502)
Cerdulatinib is a dual inhibitor of the Janus kinase (JAK) and spleen tyrosine kinase (Syk) pathways, which Dermavant is evaluating as a differentiated topical treatment option for vitiligo and other inflammatory skin conditions such as atopic dermatitis. The Phase IIa study is a multi-center, randomized, double-blind, vehicle-controlled study to assess the safety, tolerability, and systemic exposure of cerdulatinib gel 0.37%, dosed twice daily for six weeks versus vehicle in 30 adult patients aged 18-70 years diagnosed with vitiligo. The primary endpoints of the study are the safety and tolerability of topical administration of cerdulatinib gel 0.37% in adult subjects with vitiligo assessed by frequency, duration, and severity of adverse events (local and systemic), vital signs, laboratory values, and local tolerability scale (LTS) scores.
Aclaris Therapeutics, Inc. (ATI-50002/ATI-502)
ATI-502 (AA-201 Topical), is an investigational topical Janus Kinase (JAK) 1/3 inhibitor, developed by aclaris therapeutics used to treat Vitiligo. Currently, ATI-502 is evaluated under the clinical trial study of Phase II. This is a multicenter, open-label study designed to evaluate the safety, tolerability, and efficacy of ATI-50002 Topical Solution 0.46% in subjects with non-segmental facial vitiligo. Subjects would be required to have a clinical diagnosis of non-segmental facial vitiligo affecting at least 0.25% of total body surface area (TBSA) (excluding upper and lower eyelids, mucosal lip areas, and forehead and chin areas covered by the stereotactic positioning device for photography) with at least one area of the face with normal pigmentation. Twenty-four eligible subjects would receive ATI-50002 Topical Solution, 0.46%, BID for 24 weeks.
Pfizer (PF-06651600)
PF-06651600 is an oral JAK3 inhibitor used to treat Vitiligo. The JAK pathways are believed to play an important role in inflammatory processes as they are involved in signaling for over 50 cytokines and growth factors, many of which drive immune-mediated conditions. JAK inhibition may offer patients with these conditions a potential new advanced treatment option. PF-06651600 initiated a Phase II clinical trial study. This is a randomized, double-blind, parallel-group, multicenter study with an extension period. The study would have a maximum duration of approximately 60 weeks. This includes an up to 4 weeks Screening Period, a 24-week dose-ranging period, an up to 24 week extension period and an 8 week Follow up Period.
Amgen (AMG 714)
AMG 714 (PRV-015) is a human immunoglobulin monoclonal antibody that binds to IL-15 developed by amgen used to treat Vitiligo. Currently, AMG 714 is evaluated under a clinical trial study. The Phase IIa, double-blind, placebo-controlled, multi-center, proof of concept trial of AMG 714 for the treatment of vitiligo. Participants would be randomized 2:1 to receive AMG 714 or placebo for AMG714. The random assignment would be stratified by active versus stable vitiligo.
Vitiligo Emerging Pipeline Landscape
| Company Name | Product Name | Mechanism of Action | Route of Administration | Stage of Development |
| Incyte Corporation | Ruxolitinib/INCB018424 | JAK1/JAK2 Inhibitors | Topical | Phase III |
| Dermavant Sciences GmbH | Cerdulatinib 0.37% gel/DMVT-502 | JAK/Syk Inhibitors | Topical | Phase II |
| Aclaris Therapeutics, Inc. | ATI-50002 | Janus kinase 1 Inhibitors; Janus kinase 3 Inhibitors | Topical | Phase II |
| Pfizer | PF-06651600 | JAK3 Inhibitors | Oral | Phase II |
| Amgen | AMG 714 | Interleukin 15 Inhibitors | Subcutaneous | Phase II |