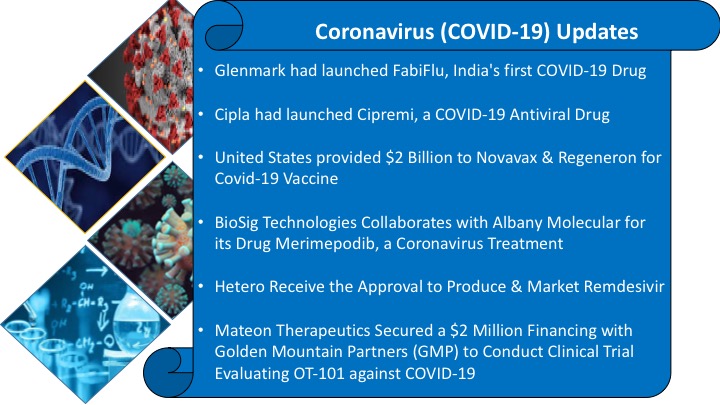
Glenmark Pharmaceuticals had launched FabiFlu, India’s first COVID-19 drug
Glenmark Pharmaceuticals had launched FabiFlu, an antiviral drug Favipiravir, to cure patients with mild to moderate coronavirus at a price tag of about Rs 103 per tablet. FabiFlu would be available in the form of a 200 mg tablet and will be at a maximum retail price (MRP) of Rs 3,500 for a strip of 34 tablets. It is the first oral favipiravir-approved medication for the treatment of COVID-19 in India. FabiFlu is a prescription-based medication with a recommended dose being at 1,800 mg twice daily on day one, followed by 800 mg twice daily up till day 14.
Cipla had launched Cipremi, a COVID-19 Antiviral Drug
Cipla had launched antiviral drug i.e., Cipremi for the treatment of COVID-19. Cipremi is available in the form of lyophilized powder (freeze dry) for injection 100mg. It has been approved for adult and pediatric patients who have been hospitalized. This drug has been granted regulatory approval by the Drug Controller General of India (DCGI) for restricted emergency use in India as part of the accelerated approval process considering the urgent and unmet medical need. The clinical trials have demonstrated the efficacy of Cipremi for the patients on oxygen support after falling ill to coronavirus.
United States provided $2 Billion to Novavax & Regeneron for Covid-19 Vaccine
The federal government awarded $2 billion to Novavax Inc. & Regeneron Pharmaceuticals for the development and manufacturing of an experimental drug and a potential vaccine against Covid-19. Novavax Inc. has received $1.6 billion from the federal government’ to fund clinical studies of its experimental coronavirus vaccine and establish large-scale manufacturing of doses. Regeneron Pharmaceuticals Inc. has received a $450 million federal contract to manufacture thousands of doses of its experimental Covid-19 treatment that the government would distribute for free to the public if the drug is authorized for use by regulators.
BioSig Technologies Collaborates with Albany Molecular for its Drug Merimepodib, a Coronavirus Treatment
BioSig Technologies had entered into the collaboration with Albany Molecular Research Inc to evaluate the merimepodib for the treatment of SARS-CoV-2, the virus that causes Covid-19. Under the terms of the collaboration, both companies would evaluate the drug as a standalone treatment and in combination with other antiviral agents or immune modulators. This collaboration would help in rapid transfer, scale-up and validation of merimepodib API manufacture as the future supply chain and commercialization strategy.
Hetero Receive the Approval to Produce & Market Remdesivir
Indian pharmaceutical firm Hetero had received approval from the Drug Controller General of India to manufacture and market the experimental antiviral drug remdesivir for the treatment of coronavirus. Hetero’s generic version of Remdesivir would be sold under the brand name Covifor in India. Covifor would be available in 100 mg vials that would be injected into the patient at a hospital facility under the proper supervision of a doctor or trained healthcare worker. The drug would be launched in India under a licensing agreement with Gilead Sciences Inc, an American biopharmaceutical firm, to expand access to Covid-19 medication in poor countries.
Mateon Therapeutics Secured a $2 Million Financing with Golden Mountain Partners (GMP) to Conduct Clinical Trial Evaluating OT-101 against COVID-19
Mateon Therapeutics had secured a $2 million in debt financing with Golden Mountain Partners (GMP) for the conduct of a clinical trial evaluating OT-101 against COVID-19. OT-101 is an antisense against the host TGF-β protein required for viral replication and its overexpression likely to cause the wide range of clinical symptoms associated with COVID-19 including Kawasaki syndrome.