The importance of diagnostic market increased significantly amid the coronavirus pandemic outbreak, by providing a new potential growth opportunity pocket which enables the diagnostic companies to launch and commercialized new product in the Indian market to save the lives of thousand people, by ramping up the diagnosis process through identification of the virus and treatment. The major drivers are lifestyle diseases rising, improving per capita, increasing awareness, and increasing payer coverage. Apart from it, they are certain influencing factors such as accessibility, lack of specialists, lack of standardization & affordability still a concern.
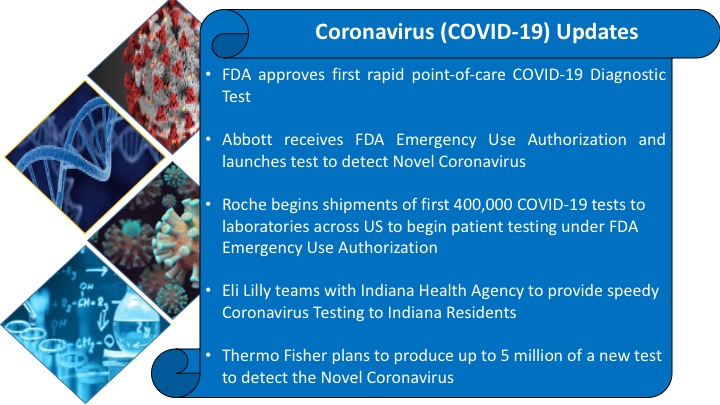
Out of many private companies, recently the government had allowed two private companies include Indian company MyLab and German firm Altona Diagnostics – to supply COVID-19 test kits to both government as well as private testing laboratories in India. In addition, two south Korean firms, Seegene and SD Biosensor have received government approval to supply RT-PCR based Novel Coronavirus (COVID-19) diagnostic kits in India.
The government has also approved 12 rapid antibody test kits for COVID-19 diagnosis companies such as BioMednomics (USA), Getein Biotech (China), Sensing Self Ltd (Singapore), Hangzhou Biotest Biotech (China), AmonMed Biotechnology Co (China), Beijing Tigsun Diagnostics Co Ltd (China), Biomaxima (Poland), CTK Biotech (USA), Hunan Lituo Biotechnology Co (China), Vivacheck Lab (China) and Wondfo (China). While, The rapid antibody test facilitate result within 30 minutes, thereby providing additional benefits. The test comes positive after 7 to 10 days of infection. While a positive test indicates exposure to COVID-19, negative does not rule out the infection.
There are several companies issued a CDSCO license in the Indian market such as Altona Diagnostics, MY LABS, Roche & Seegene, etc.
Altona Diagnostics: RealStar SARS-CoV-2 RT-PCR Kits
Altona Diagnostics, RealStar SARS-CoV-2 RT-PCR Kits (1.0 and U.S versions), is a reagent system, based on real-time PCR technology, for the qualitative detection and differentiation of lineage B-betacoronavirus (B-βCoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) specific RNA. These tests can be run in parallel: RealStar® Influenza Screen & Type RT-PCR Kit 4.0.etc
Mylab Discovery Solutions Pvt Ltd: PathoDetect COVID-19 Qualitative PCR ‘Made in India’ test kits
Pune based Mylab Discovery Solutions Pvt Ltd developed PathoDetect COVID-19 Qualitative PCR ‘Made in India’ test kits are capable of diagnosing a patient in two-and-a-half hours, unlike the imported kits, which currently take up to seven hours. The kits have reportedly been developed in a record time of six weeks and will reduce the cost of testing to a fourth of the current cost. It also the first Indian diagnostic kit approved by the ICMR against COVID-19.
Allplex: 2019-nCoV Assay
Allplex 2019-nCoV Assay is a multiplex Real-time PCR assay for simultaneous detection of 3 target genes of SARS-CoV-2 in a single tube. The assay is designed to detect RdRP and N genes specific for SARS-CoV-2, and E gene for all of Sarbecovirus including SARS-CoV-2. It shows PCR with high sensitivity and specificity by giving results within 1 hour and 50 minutes after extraction. It has also inserted an automatic data analyzer and LIS interlocking with Seegene Viewer. It also gives quick response and proper treatment provided by accurate test results.
Genesig: Real-Time PCR COVID-19
Genesig Real-Time PCR COVID-19 (CE) is CE-IVD marked and intended for in vitro diagnostic use in Europe. This is a rapid kit detection and exclusive to the COVID-19 strain, however not involved in the detection of other related coronavirus strains. It has high priming efficiency and a highly specific detection profile. Besides, Lyophilised components for ambient shipping. It also involved in driving accurate controls to confirm extraction and assay validity.
Roche Diagnostics: Cobas SARS CoV-2 Diagnostic Test Kit
Roche Diagnostics India has received the license from the country’s drug regulator DCGI for its ‘cobas SARS CoV-2’ diagnostic test kit, making it the first private firm to get permission to conduct coronavirus tests Since, the NIV has tested 14 privately-made kits and recommended three kits made Atona Diagnostics, MyLab, and Seegene for CDSCO license.
Commercialized Diagnostic Products
| Company | Diagnostic Kit | Product Description |
| Altona diagnostic | RealStar SARS-CoV- 2 RT-PCR kit 1.0 | Reagent system, based on real-time PCR technology, for the qualitative detection and differentiation of lineage B-betacoronavirus (B-βCoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) specific RNA. These tests can be run in parallel |
| MY LAB | Patho Detect | PathoDetect COVID-19 Qualitative PCR kit would cost just around one-fourth of the current procurement cost. |
| BGI | Real Time Fluorescent RT-PCR kit for detecting 2019-nCoV | Reagents commercially distributed as a kit to labs Can only be run in high complexity labs |
| Krishgen Biosystem | SARS-CoV-2 Coronavirus Real-Time RT-PCR (RT-Qpcr) Detection kit v1 | Uses Taqman probes. Taqman Probes are widely used RT-PCR assay because of its sensitivity and specificity with dual quenchers for higher accuracy. With 2/3 Primer/Probe option |
| ABI | TaqMan 2019-nCov Control kit v1 | TaqMan 2019-nCoV Control Kit v1 is a synthetic positive control that contains target sequences for each of the assays included in the TaqMan 2019-nCoV Assay Kit v1 (Cat. No. A47532). The control includes synthetic DNA target sequences for three SARS-CoV-2 genes (ORF1ab, S protein, and N protein) and the human RNase P RPPH1 gene. |
| HIMEDIA | Hi-PCR Coronavirus (CoVID-19) Probe PCR kit | To perform PCR on various DNA templates for numerous downstream applications. It also contains all necessary components including HiMedia’s ultra-efficient Taq Polymerase, dNTP mixture, and MgCl2 for reproducible PCR results. |
| HUWEL | Quantiplus Coronavirus (2019nCoV) Detection kit | The kit has a synthetic gene cloned in a plasmid as a positive control. The primers set is cross-verified for specificity with several RNA and DNA viruses and bacteria |
| IIT-DELHI | SYBR Green based one step QRT-PCR | First probe-free assay for COVID-19 approved by the ICMR and it will be useful for specific and affordable high throughput testing. It can easily be scaled up as it does not require fluorescent probes. Targeting large-scale deployment of the kit at affordable prices with suitable industrial partners as soon as possible. |
| KILPEST (BLACKBIO) | TRUPCR | Real-Time PCR Test is a molecular detection test that screens and detects Covid-19 specific genes and is confirmatory. |
| Genesig | Coronavirus (COVID-19) genesig Real Time PC R Assay | Rapid detection and exclusive to the COVID-19 strain Does not detect other related coronavirus strains High priming efficiency |
| Roche | LightMix Modular SARS and Wuhan CoV E gene | E_Sarbeco_F1 (Oligonucleotide ID) Contains ACAGGTACGTTAATAGTTAATAGCGT (5’-3’ sequence ) by using use 400 nM per reaction |
| Roche | LightMix Modular SARS and Wuhan CoV N gene | N_Sarbeco_F1 (Oligonucleotide ID) Contains CACATTGGCACCCGCAATC (5’-3’ sequence ) by using use 600 nM per reaction |
| Roche | LightMix Modular Wuhan RdRp gene | Detect Wuhan 2019 SARS-like CoV pneumonia virus Sensitivity is 3.8 copies per reaction (95% CI: 2.7–7.6) Lot release min 10 copies |
| Seegene | Allplex 2019-nCoV assay | Multiplex real-time PCR assay for simultaneous detection of 3 target genes of SARS-CoV-2 in a single tube Results within 1 hour and 50 minutes after extraction |