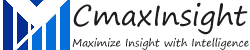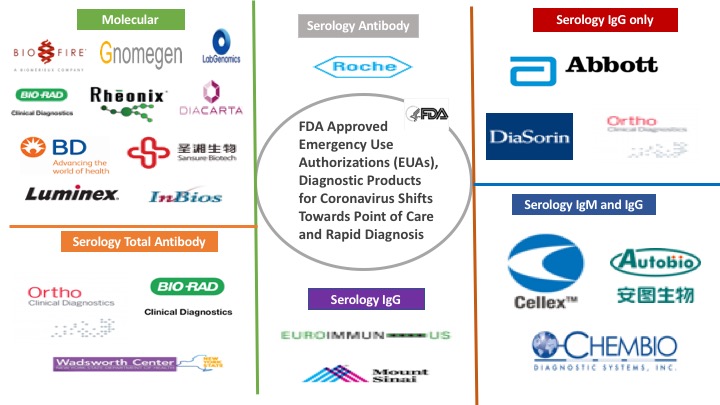
Moderna Vaccine’s Progress, Excites COVID-19 Markets
Moderna Inc’s experimental COVID-19 vaccine, the first to be tested in the United States, produced protective antibodies in a small group of healthy volunteers. However, the data comes from eight people who took part in a 45-subject safety trial that kicked off in March. Afterward, Phase I trial results of the vaccine, mRNA-1273, have been found promising. On May 7, the company has received regulatory clearance for the Phase II trial that would involve a much larger sample size. The imminent Phase II study start is a crucial step forward as we continue to advance the clinical development of mRNA-1273, our vaccine candidate against SARS-CoV-2. With the goal of starting the mRNA-1273 pivotal Phase III study early this summer, Moderna is now preparing to potentially have its first BLA approved as soon as 2021. In addition, Moderna launched a $1.34 billion share offering at an offer price of $76 per share. The company had earlier said it plans to sell $1.25 billion in common stock to raise money for vaccine development and manufacturing.
Canada Approves the First Clinical Trial for potential Covid-19 Vaccine
The race is on to find a vaccine for COVID-19 and Canada’s government may have taken the lead after its national drug regulator cleared a plan to begin studying the front-running candidate. This investigation drug is an adenovirus-based vaccine developed jointly by China’s CanSino Biological and the National Research Council of Canada and is one of eight to have made it to the clinic, and is already in phase II development in China.
Gilead Signs Licensing Agreement With Cipla, Jubilant Life, Mylan, Hetero Labs & Ferozsons Labs
Gilead signed non-exclusive licensing agreements with five companies based out of India and Pakistan for their investigational drug remdesivir, to treat COVID-19 patients. Gilead signed licenses with Cipla, Jubilant Lifesciences, Hetero Labs, Mylan, and Ferozsons Labs. Under the agreement for remdesivir, Gilead would transfer its technology on how the API and formulations are developed to the companies and distribute it in 127 countries including India.
The deal would ensure accessibility and affordability of remdesivir in especially lower income countries with poor health infrastructure. Besides India, this license agreement extends to countries such as Afghanistan, Bhutan, Cambodia, South Africa and Egypt to name a few. It does not extend to larger and more developed countries such as US, UK, Europe, China, Australia amongst others. Hence, the drug was given emergency use authorization (EUA) by the USFDA for treating COVID-19 patients, but is yet to be formally approved by the drug regulator. Further, this is the second voluntary license agreement that Gilead has signed.
China Tests Coronavirus Drug That Aims to Stop Pandemic Sans Vaccine
A Chinese laboratory has been developing a drug it believes has the power to bring the coronavirus (COVID-19) pandemic to a halt. A drug being tested by scientists at China’s prestigious Peking University could not only shorten the recovery time for those infected but even offer short-term immunity from the virus. It is isolated from the blood of 60 recovered patients. The drug has been successful at the animal testing stage. The injection of the neutralizing antibodies into infected mice resulted in reducing the viral load was reduced by a factor of 2,500 This potential drug has a therapeutic effect.
Oxford’s Jenner Institute & AstraZeneca Collaborate Together
Oxford’s Jenner Institute had entered into the collaboration with the AstraZeneca for the development of the vaccine. The experimental vaccine, known as ChAdOx1 nCoV-19, is one of the front runners in the global race to provide protection against the new coronavirus causing the COVID-19 pandemic.
Microsoft & UnitedHealth Launch COVID-19 Screening App For The Workplace
Microsoft and UnitedHealth Group are launching a free smartphone app that businesses and employees can use to digitally screen for COVID-19 symptoms and clear those who can return to work. Based on guidelines from the Centers for Disease Control and Prevention, the ProtectWell app uses Microsoft’s chatbot interface to ask the user a series of daily questions, such as whether they have had a fever or any respiratory symptoms or whether they’ve been in contact with anyone diagnosed with COVID-19.
Fitbit to Develop Emergency COVID-19 Ventilator
Fitbit aims to wield its fitness-focused supply chain to start building its own COVID-19 ventilator and plans to submit its designs to the FDA in the near future. This device would include more features than the typical emergency alternatives developed in response to the spreading coronavirus pandemic, which are largely made to be built quickly and at scale using off-the-shelf components and mechanically deliver puffs of air at a fixed rate.