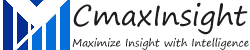June is the Scoliosis Awareness Month that aims at highlighting the growing need for education, early detection, and awareness regarding scoliosis and its prevalence within the community. It unites scoliosis patients, families, physicians, clinicians, institutions, and related businesses in collaborative partnerships of local activities, events, and grassroots networking throughout the month.
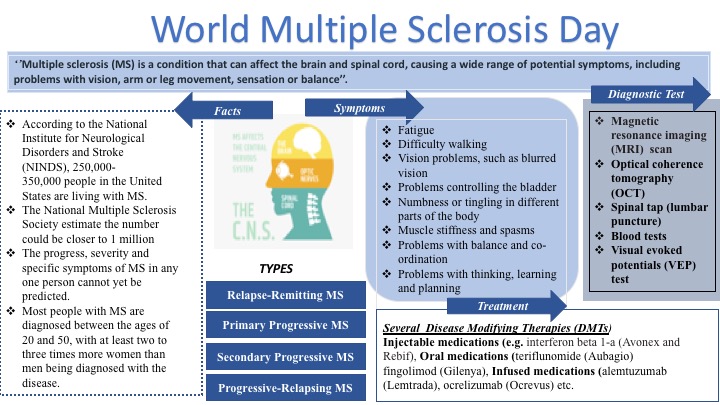
Scoliosis Overview
Scoliosis is an abnormal side-to-side curvature of the spine. The spinal curve may develop as a single curve (shaped like the letter C) or as two curves (shaped like the letter S). It is often defined as spinal curvature in the “coronal” (frontal) plane. While the degree of curvature is measured on the coronal plane, scoliosis is actually a more complex, three-dimensional problem which involves the following planes:
- Coronal plane
- Sagittal plane
- Axial plane
The coronal plane is a vertical plane from head to foot and parallel to the shoulders, dividing the body into anterior (front) and posterior (back) sections. The sagittal plane divides the body into right and left halves. The axial plane is parallel to the plane of the ground and at right angles to the coronal and sagittal planes.
Scoliosis is hereditary among the people with scoliosis who are more likely to have children with scoliosis; however, there is no correlation between the severity of the curves from one generation to the next. In children and teens, scoliosis often does not have any noticeable symptoms and may not be noticeable until it has progressed significantly. Most cases of scoliosis are mild, but some spine deformities continue to get more severe as children grow. Severe scoliosis can be disabling. An especially severe spinal curve can reduce the amount of space within the chest, making it difficult for the lungs to function properly. The most common form of scoliosis appears in adolescents. It is known as adolescent idiopathic scoliosis. It can affect children from the age of 10 years.
The symptoms can include the head is slightly off center, the ribcage is not symmetrical, so the ribs may be at different heights and one hip is more prominent than the other. Furthermore, in infants, symptoms can include: a bulge on one side of the chest, consistently lying curved to one side (in babies), Problems with the heart and lungs, leading to shortness of breath and chest pain.
According to the American Association of Neurological Surgeons (AANS), about 80 percent of scoliosis cases have no identifiable cause. The condition is often diagnosed during the first seven years of a child’s life.
Etiology
- Neuromuscular Conditions: These affect the nerves and muscles and include cerebral palsy, poliomyelitis, and muscular dystrophy.
- Congenital Scoliosis (present at birth): This is rare and occurs because the bones in the spine developed abnormally when the fetus was growing inside the mother.
- Specific genes: At least one gene is thought to be involved in scoliosis.
- Leg length: If one leg is longer than the other, the individual may develop scoliosis.
- Syndromic scoliosis: Scoliosis can develop as part of another disease, including neurofibromatosis and Marfan’s syndrome.
- Osteoporosis: This can cause secondary scoliosis due to bone degeneration.
Risk Factors
There are certain risk factors associated with scoliosis include: age, gender & genetics etc. are explained below:
- Age: Signs and symptoms often start during a growth spurt just before puberty.
- Gender: Females have a higher risk in comparison to the males
- Genetics: People with scoliosis may have a close relative with the condition.
Diagnosis
Scoliosis is confirmed through a physical examination, an x-ray, spinal radiograph, CT scan or MRI. The curve is measured by the Cobb Method and is diagnosed in terms of severity by the number of degrees. A positive diagnosis of scoliosis is made based on a coronal curvature measured on a posterior-anterior radiograph of greater than 10 degrees. In general, a curve is considered significant if it is greater than 25 to 30 degrees. Curves exceeding 45 to 50 degrees are considered severe and often require more aggressive treatment.
A standard exam that is sometimes used by pediatricians and in grade school screenings is called the Adam’s Forward Bend Test. During this test, the patient leans forward with his or her feet together and bends 90 degrees at the waist. This is a simple initial screening test that can detect potential problems, but cannot determine accurately the exact type or severity of the deformity. The tests are required for an accurate and positive diagnosis.
Physical Examination
Doctor would check the spine curvature and whether the shoulders and waist area are symmetrical or not.
Imaging Tests
Imaging tests doctor may order to look for scoliosis include:
- X-ray: During this test, small amounts of radiation are used to create a picture of your spine.
- MRI scan: This test uses radio and magnetic waves to get a detailed picture of bones and the tissue surrounding them.
- CT scan: During this test, X-rays are taken at a variety of angles to get a 3-D picture of the body.
- Bone scan: This test detects a radioactive solution injected into your blood that concentrates in areas of increased circulation, highlighting spinal abnormalities.
Treatment
Treatment of scoliosis is based on the severity of the curve and the chances of the curve getting worse. Certain types of scoliosis have a greater chance of getting worse, so the type of scoliosis also helps to determine the proper treatment. There are three main categories of treatment i.e. observation, bracing (for example, thoracolumbosacral orthosis or TLSO back brace), and surgery. Consequently, there are treatments available that do not involve surgery, but in some individuals, surgery may be their best option.
Observation
In many children with scoliosis, the spinal curve is mild enough to not require treatment. However, if the doctor is worried that the curve may be increasing, he or she may wish to examine the child every four to six months throughout adolescence.
In adults with scoliosis, X-rays are usually recommended once every five years, unless symptoms are getting progressively worse.
Bracing
Braces are only effective in patients who have not reached skeletal maturity. If the child is still growing and his or her curve is between 25 degrees and 40 degrees, a brace may be recommended to prevent the curve from progressing. There have been improvements in brace design and the newer models fit under the arm, not around the neck. There are several different types of braces available. For optimal effectiveness, the brace should be checked regularly to assure a proper fit and may need to be worn 16 to 23 hours every day until growth stops.
Surgery
In children, the two primary goals of surgery are to stop the curve from progressing during adulthood and to diminish spinal deformity. Most experts would recommend surgery only when the spinal curve is greater than 40 degrees and there are signs of progression. This surgery can be done using an anterior approach (through the front) or a posterior approach (through the back) depending on the particular case.
A number of factors can lead to increased surgical-related risks in older adults with degenerative scoliosis. These factors include the following: advanced age, being a smoker, being overweight and the presence of other health/medical problems. In general, both surgery and recovery time are expected to be longer in older adults with scoliosis.
Following surgical procedures are used for the treatment of scoliosis
- Posterior approach: The most frequently performed surgery for adolescent idiopathic scoliosis involves posterior spinal fusion with instrumentation and bone grafting. This is performed through the back while the patient lies on his or her stomach.
- Anterior approach: The patient lies on his or her side during the surgery. The surgeon makes incisions in the patient’s side, deflates the lung and removes a rib in order to reach the spine. Video-assisted thoracoscopic (VAT) surgery offers enhanced visualization of the spine and is a less invasive surgery than an open procedure. The anterior spinal approach has several potential advantages: better deformity correction, quicker patient rehabilitation, improved spine mobilization and fusion of fewer segments.
- Decompressive laminectomy: The laminae (roof) of the vertebrae are removed to create more space for the nerves. A spinal fusion with or without spinal instrumentation is often recommended when scoliosis and spinal stenosis are present. Various devices (like screws or rods) may be used to enhance fusion and support unstable areas of the spine.
- Minimally invasive surgery (MIS) : Fusion can sometimes be performed via smaller incisions through MIS. The use of advanced fluoroscopy (X-ray imaging during surgery) and endoscopy (camera technology) has improved the accuracy of incisions and hardware placement, minimizing tissue trauma while enabling a MIS approach. It is important to keep in mind that not all cases can be treated in this manner and a number of factors contribute to the surgical method used.
The benefits of surgery should always be weighed carefully against its risks. Although a large percentage of scoliosis patients benefit from surgery, there is no guarantee that surgery will stop curve progression and symptoms in every individual.